It has been an alarming week for those following Britain’s reaction to the pandemic. Entirely disparaged for the delay of its lockdown and its screwed up testing programs, the UK was the unforeseen beneficiary of an unexpected episode of extravagant commendation for its researchers’ endeavors to battle the infection.
“The Brits are on course to spare the world,” composed driving US financial expert Tyler Cowen in Bloomberg Opinion, while the diary Science cited driving worldwide researchers who have stored acclaim on British specialists’ enemy of Covid work.
What’s more, the practical objective for these approvals has been the UK’s Recovery preliminary, a medication testing program that has included contribution from in excess of 3,000 specialists and attendants working with 12,000 Covid-19 patients in 176 emergency clinics the country over – from the Western Isles to Truro and from Derry to King’s Lynn. These preliminaries were done in concentrated consideration units packed with the truly sick, patients whose numbers were supported to significant levels due to the UK’s late pandemic lockdown. The outcomes have in any case changed Covid-19 clinical practice over the planet.
A modest irritation treatment has been found to spare the lives of truly sick patients while two much-promoted treatments have been demonstrated to be futile at handling the sickness. No other country has verged on coordinating these accomplishments.
“It has been an uncommon four months,” says one of Recovery’s originators, Martin Landray of Oxford University. “Also, indeed, it is something that the UK can be pleased with.”
Landray is a specialist in setting up enormous clinical preliminaries while his fellow benefactor, Peter Horby, additionally of Oxford University, is an irresistible ailment expert who was associated with Covid medicate preliminaries in Wuhan the previous winter when the pandemic originally rose. Be that as it may, these investigations finished when case numbers plunged as the Chinese specialists applied their unbending lockdown. “Simultaneously, cases started showing up in Europe and I understood we have to begin work here,” says Horby.
So he and Landray united and set up Recovery, short for Randomized Evaluation of Covid-19 Therapy. “We understood specialists would before long be searching for medicines once cases began pouring in to our emergency clinics,” says Landray. “On the off chance that we didn’t rapidly begin preliminaries, we could never know whether the medications that we utilized were any acceptable. We had around fourteen days to get a program ready for action before the wave hit the NHS.”
The pair took nine days from drafting their first convention to selecting their first patient. “Typically it takes nine months to do that,” says Horby. “For good measure, we enlisted 10,000 patients inside about two months.”
Randomized medication preliminaries are the best quality level for pinpointing valuable drugs, expelling oblivious inclinations that can cloud clinicians’ decisions. A large number of individuals are given a medication or a fake treatment. Nobody knows which they are taking. At that point results are looked at and the adequacy of the treatment is uncovered.
Essentially, these preliminaries need enormous quantities of patients and no single medical clinic has enough for such examination. England has one key bit of leeway, in any case, in that it has an incorporated National Health Service. Different countries, specifically the US, have wellbeing administrations that are divided. “It’s the foundation in this nation – the NHS and the National Institute of Health Research, who subsidized Recovery – that has made this potential,” says Horby. A quarter of a year in the wake of setting up their tasks, Recovery delivered its first outcomes. The first was for the counter malarial medication hydroxychloroquine, which was then being broadly promoted by legislators, for example, Donald Trump, Emmanuel Macron and Jair Bolsonaro as a successful Covid-19 treatment. All trusted it could spare the world.
The Recovery group deviated, be that as it may. Their preliminaries indicated hydroxychloroquine gave no assistance to Covid patients of any sort. After ten days, the medication’s Emergency Use Authorisation in the US was pulled back – to the disturb of Trump.
It was a significant turn of events, says Landray. “It implied medical clinics around the globe not, at this point expected to squander assets on a futile treatment, and could stop erroneously raising patients’ desire.”
At that point there was the mix treatment of lopinavir and ritonavir, two enemy of HIV specialists, which was likewise being promoted as a ground-breaking Covid treatment. Recuperation analyzed 1,596 patients who were given the medications with 3,376 who were not – and found no noteworthy distinction in death rates between the two gatherings. It was another mistake.
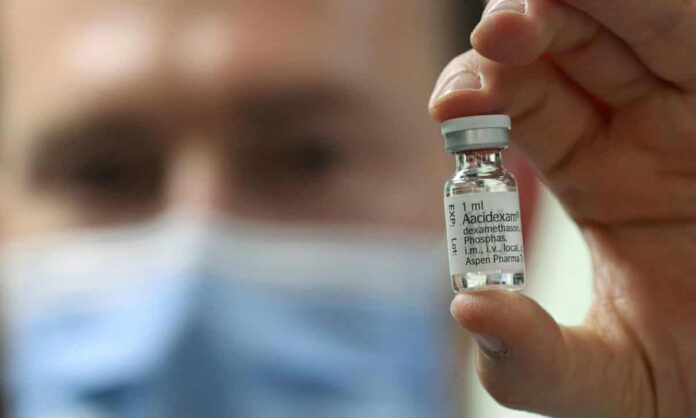
At long last, Recovery went to dexamethasone, a modest steroid used to counter irritation and treat joint inflammation. This was appeared to decrease passings by a third among patients on ventilators in serious consideration units. “This is a medication that costs £5 a course – close to nothing – and is broadly accessible,” says Landray. “It was a mind blowing shock and an enormous advance forward.”
Thus dexamethasone has been added to the clinical direction notes for rewarding seriously sick Covid-19 patients in emergency clinics around the globe while lopinavir and ritonavir just as hydroxychloroquine have been evacuated. “Clinicians think of a wide range of theories about the value of medications,” says Landray. “However, you must test these theories and to do so you need enormous, randomized preliminaries.”
The estimation of this methodology has been noted on the opposite side of the Atlantic. Recuperation’s three preliminaries are the best to have been performed anyplace on the planet, Eric Topol, executive of the Scripps Research Translational Institute told the diary Science this month.
Two other, far more troubled, impacts have likewise affected Recovery’s prosperity, be that as it may. “Right off the bat, we had an extremely large plague in the UK thus had a ton of patients who we could select,” says Horby. “The other was the high passing rate among the individuals who were hospitalized, around 25%. On the off chance that that death rate had been just 5% we would have required a much bigger preliminary to deliver important outcomes.”
Current preliminaries are presently concentrating on three other up-and-comer medicines: an anti-toxin called azithromycin, a counter acting agent called tocilizumab and a treatment known healing plasma – blood plasma that is taken from individuals who have had coronavirus and which will contain antibodies that may help the individuals who are truly influenced by the infection. In any case, Horby and Landray state it might take a very long time to get results from these outcomes. “Less individuals are currently being hospitalized – which implies less individuals are presently being enlisted to Recovery,” says Landray. “That implies results which were quickly accomplished half a month prior will presently take longer. It’s uplifting news, truly.”
Altered by NZ Fiji Times
Image source - The Guardian